Abstract
Background: Malignant lymphoma, represented by intravascular large B-cell lymphoma (IVLBCL), is an important differential disease in classical fever of unknown origin (FUO). Bone marrow aspiration (BMA) and random skin biopsy (RSB) are useful in IVLBCL diagnosis, but their positivity rates are clinically insufficient and the procedures are invasive. The molecular genetic analysis of lymphomas using cell-free tumor DNA (cfDNA) in plasma (liquid biopsy) may be effective for IVLBCL (Shimada et al., Blood. 2021 Mar 18;137(11):1491). We conducted a multicenter prospective clinical trial to investigate the usefulness of liquid biopsy for early lymphoma diagnosis among patients with classical FUO.
Methods: Patients with the following criteria were enrolled: (1) clinically suspected malignant lymphoma, (2) persistent fever for >3 weeks, refractory to antibiotics, (3) higher than standard lactate dehydrogenase, (4) unbiopsiable lesions, and (5) age ≥20 years. Liquid biopsies were performed as follows: (1) MYD88 L265P/CD79 Y196 mutation analysis using droplet digital PCR (ddPCR), (2) B-cell receptor (BCR)/T-cell receptor (TCR) reconstruction analysis using next generation sequencing (NGS), and (3) comprehensive genetic analysis using the hematopoietic oncogene panel (426 genes) and NGS. Pathological malignant lymphoma was diagnosed at each institution; later, a central diagnosis was made by two additional lymphoma pathology specialists.
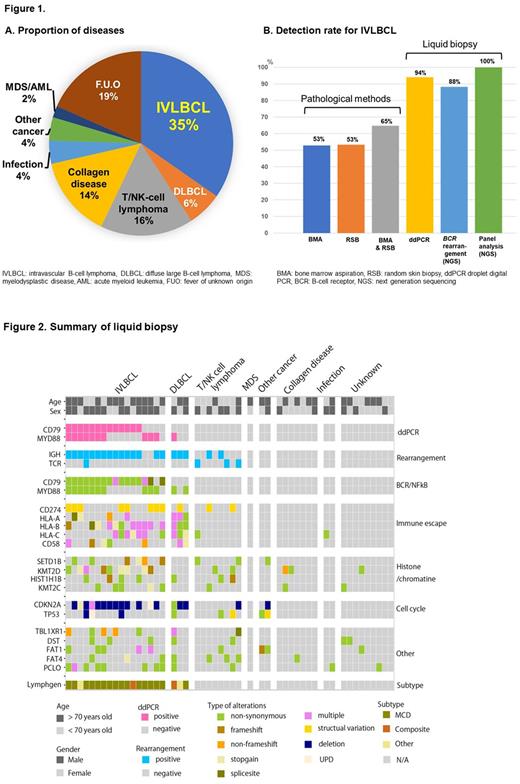
Results: Fifty patients were enrolled from 15 centers from October 2019 to September 2021; one was excluded. The median age was 73.5 (21-89) years; 28 patients (57.1%) were male. There were 29 cases (59%), 28 (57.1%), and one (2%) of hematologic malignancy, malignant lymphoma, and MDS/AML, respectively (Fig. 1A). Twenty (71%) malignant lymphomas were B-cell lymphomas (17 of which were IVLBCL [61%]), and 8 (29%) were mature T/NK-cell lymphomas. Nonhematologic diseases were present in 20 (41%) patients (7 collagen diseases [4 adult-onset Still's disease, 3 others], 2 solid tumors, 2 infections [1 syphilis, 1 tuberculosis], and 9 FUO). FUO pathological investigations were performed in 47 (97.9%) and 43 (87.6%) patients with BMA and RSB, respectively. IVLBCL had a 53% positivity rate for initial BMA (9/17) and RSB (8/15; Fig. 1B). Only 65% patients tested positive for either initial BMA or RSB and a majority (15/17) of them were diagnosed based on repeated evaluations during their lifetime. The BMA positive rate in patients with mature T/NK-cell lymphoma (8 cases) was high (75%). The mean cfDNA concentration was 215 (5.6-1320) ng/mL, with no significant difference between patients with lymphoma and those with other diseases (279 vs. 130 ng/mL, p=0.073). In ddPCR analysis, 85% of B-cell lymphoma cases (N=20) had either MYD88 or CD79B mutations; the mutation detection rate in IVLBCL (N=17) was 94% (Fig.1B, 2). All patients with non-B-cell lymphoma tested negative. The positive rate of patients with B-cell lymphoma in the NGS-based BCR reconstruction analysis was 90% and negative in two patients with IVLBCL. BCR reconstitution was also observed in two patients with T/NK-cell lymphoma. Comprehensive panel analysis demonstrated that 19/20 (95%) B-cell lymphoma cases showed MYD88 or CD79B mutations; all IVLBCL cases showed mutations. LymphGen algorithm analysis showed that 15, 1, and 1 IVLBCL cases were categorized as MCD (with MYD88 or CD79b mutations), composite (including MCD), and other, respectively (Fig. 2). In T/NK-cell lymphomas, TCR reconstitution was positive in 3/8 T/NK-cell lymphomas. Panel analysis could hardly predict T/NK-cell lymphoma. DdPCR results were returned to the physician within a median time of 14 days. Of the six patients who were not diagnosed with initial BMA and RSB, five were positive for the MYD88/CD79B mutation, according to the ddPCR results. Three of them were diagnosed through additional liquid biopsy testing. Two patients were diagnosed with IVLBCL based on central diagnosis after death.
Conclusions: In the classical FUO cases with suspected malignant lymphoma, 57% and 35% had malignant lymphoma and IVLBCL, respectively. Compared to the classical diagnostic methods, liquid biopsy can be used to detect lymphoma-specific genetic mutations at an earlier stage, being clinically useful for the early diagnosis of difficult-to-diagnose malignant B-cell lymphomas.
Disclosures
Sanada:Otsuka pharmaceutical: Research Funding. Yasuda:Chugai Pharmaceutical: Research Funding; Nippon Shinyaku: Research Funding. Masaki:Kyowa Kirin Pharma: Research Funding; Astellas Pharma: Research Funding; Eisai Pharma: Research Funding; Asahi Kasei Pharma: Research Funding; Daiichi-Sankyo Pharma: Research Funding; Taisho Pharma: Research Funding; Takeda Pharma: Research Funding; Chugai Pharma: Research Funding; Teijin Pharma: Research Funding; Japan Blood Product Organaization: Research Funding. Miyazaki:Takeda Pharmaceutical: Honoraria; Novartis Pharma: Honoraria; AbbVie: Honoraria; Meiji Seika: Honoraria; Bristol-Myers Squibb: Honoraria; Symbio: Honoraria; Janssen Pharmaceutical: Honoraria; Nippon-Shinyaku: Honoraria; Celgene: Honoraria; Eisai: Honoraria; Chugai Pharmaceutical: Honoraria, Research Funding; AstraZeneca: Honoraria, Research Funding; Zenyaku Kogyo: Research Funding; Kyowa Kirin: Honoraria, Research Funding. Tomita:Chugai Pharmaceutical: Honoraria, Research Funding; Takeda Pharmaceutical: Honoraria; Nippon Shinyaku: Honoraria; Ono Pharmaceutical: Honoraria; Eisai: Honoraria; Kyowa Kirin: Research Funding; Perseus Proteomics: Research Funding; Pfizer Japan: Research Funding; Novartis Pharma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal